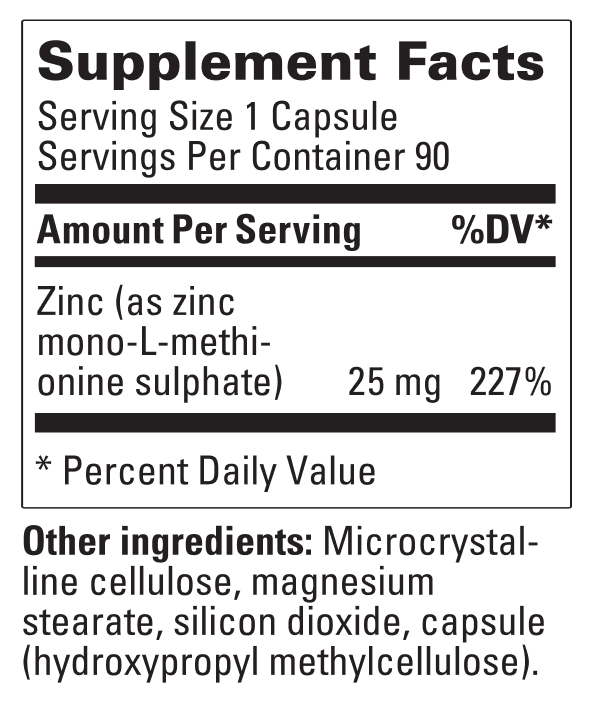


A bioavailable source of zinc in the form of a chelate compound of zinc with the amino acid methionine.
Zinc
Zinc is one of the most common trace elements in the human body. It is primarily found in bones and muscles, and it is also present in the skin, retinas of the eyes, brain, liver, pancreas, and prostate glands. Additionally, zinc is a component of many enzymes necessary for various biochemical reactions, influencing antioxidant protection, digestive processes, metabolism of proteins, fats, and carbohydrates, tissue regeneration, development and function of immune cells, synthesis of hemoglobin, and much more.
The absorption of zinc is closely related to the diet. Despite an adequate amount of dietary sources of zinc (meat, poultry, dairy products, seafood, eggs), there are also numerous foods that significantly inhibit its absorption when taken together. These include grains, legumes, nuts, seeds, as well as some cereals, fruits, and vegetables containing natural substances called phytates. These phytates form compounds with zinc that are unavailable for absorption by the body.
Chelating zinc with the amino acid methionine helps protect zinc from factors that reduce its bioavailability and improves its absorption.
*Chelates are compounds of minerals with organic molecules, often amino acids. Chelated compounds have a unique structure that determines their stability and enhances absorption by the
body.
**Compared to inorganic forms of zinc.